
NEUROSCIENCE:
Seeking
Categories in the BrainSimon J. Thorpe and Michèle
Fabre-Thorpe*
Perceptual categorization is a fascinating
cognitive operation in which the mammalian brain groups together
objects that share common properties, regardless of their physical
differences. For example, we naturally group together cats, fish,
birds, insects, and snakes into the category "animal," even though
visually they are very diverse. Understanding categorization is a
major challenge facing cognitive neuroscientists, a challenge that
Freedman and co-workers (1)
take on in their study on page 312
of this issue.
These authors examined the responses of neurons in the prefrontal
cortex (PFC) of monkeys trained to categorize animal forms
(generated by computer) as either "doglike" or "catlike." By
continuously "morphing" the basic form of one animal into the other,
the authors were able to test (with single-cell recording
electrodes) how monkey PFC neurons responded to forms that could be
either cat or dog (that is, shapes that were somewhere between the
two animals). They report that many PFC neurons responded
selectively to the different types of visual stimuli belonging to
either the cat or the dog category and with the same strength,
regardless of how morphologically close the images were to the other
category. The firing of impulses by PFC neurons thus reflects
category membership rather than simple processing of the physical
characteristics of the images.
The neurons that Freedman et al. recorded from almost
certainly receive their visual inputs from the inferior temporal
cortex (ITC), a part of the brain that lies at the end of the chain
of visual processing stages of the so-called ventral visual pathway
(see the figure). It has been known for many years that some ITC
cells can be highly selective to particular visual stimuli such as
faces (2,
3)
and can even respond to a range of two-dimensional views of the same
object (4).
More recently, Vogels examined the responses of ITC cells in monkeys
trained to categorize pictures of trees and fish. He reported a
number of cells that were only activated by certain stimuli
belonging to a given category (5),
although none of them responded to all exemplars of the category. In
a particularly impressive recent study, Sheinberg and Logothetis
recorded the activity of ITC neurons in monkeys trained to search a
large color photograph for small hidden figures--very much like the
"Where's Waldo" game familiar to children (6).
A wide range of different objects was artificially divided into two
sets. To get a reward, the monkey had to pull a lever on the left
for one set and on the right for the other set. The monkeys were
extremely good at the task, and many ITC neurons showed a strong
burst of firing when the monkey's eyes landed on (or close to)
particular targets, remaining silent while the monkey was exploring
the rest of the natural scene. However, there was no obvious
relation between the set of targets to which the neuron responded
and the artificial object categories as defined by the two response
sets. It thus appears that the cognitive task of the ITC cells may
be different from that of the PFC neurons described by Freedman
et al.--activity patterns in the Freedman monkey neurons
changed when the same set of images needed to be categorized in a
different way. Clearly we need experiments that directly compare ITC
and PFC responses using the same behavioral tests. Nevertheless, it
looks like ITC and PFC may have different parts to play in these
higher order visual tasks: ITC may provide highly processed visual
information concerning the visual objects that are present, but PFC
may be required to decide how these objects should be categorized.
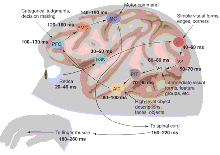
From input to output. Monkeys can
categorize complex visual stimuli very quickly, with reaction times
that average 250 to 260 ms but that can be as short as 180 ms.
Depicted is a plausible route between the retina and the muscles of
the hand during a categorization task. Information from the retina
is relayed by the lateral geniculate nucleus of the thalamus (LGN)
before reaching V1, the primary visual cortex. From there,
processing continues in areas V2 and V4 of the ventral visual
pathway before reaching visual areas in the posterior and anterior
inferior temporal cortex (PIT and AIT), which contain neurons that
respond specifically to certain objects. The inferior temporal
cortex projects to a variety of areas, including the prefrontal
cortex (PFC), which contains the visually responsive neurons that
categorize objects (1).
To reach the muscles in the hand, signals probably need to pass via
the premotor cortex (PMC) and primary motor cortex (MC) before
reaching the motor neurons of the spinal cord. For each processing
stage, two numbers (in milliseconds) are given: The first is an
estimate of the latency of the earliest neuronal responses to a
flashed stimulus, whereas the second provides a more typical average
latency.
CREDIT: CARIN CAIN
In a way, this distinction between the visual representations
seen in ITC and the more behaviorally relevant activity in PFC is
reminiscent of some much earlier work on visual responses to food.
These studies showed that neurons in the lateral hypothalamus (an
area in the limbic system involved in the control of feeding) can
respond to all stimuli that the monkey treats as food (7).
In contrast, neurons in ITC, which probably provide the input for
these food-selective neurons, fail to show such category-relatedness
(8)
despite their considerable stimulus specificity.
One of the most impressive features of visual responses seen in
both PFC and ITC is their speed. In ITC, neurons start to respond
about 100 ms after stimulus onset, and in PFC typical onset
latencies are only slightly longer. Although 100 ms may seem like a
fair amount of time, it is not very long when one takes into account
the number of processing stages involved (see the figure).
Information from the retina reaches the primary visual cortex, area
V1, via the thalamus, and is subject to further processing in areas
V2 and V4 before reaching the various parts of ITC and then PFC.
Response properties become more and more complex as one moves along
this ventral cortical stream, and onset latency increases in a
fairly systematic way, with an increase of roughly 10 ms per stage.
This does not allow much time for complex iterative processing and
suggests that the initial activation of cells in ITC and PFC could
depend largely on a feedforward pass through the visual system. Oram
and Perrett provided support for such a view by showing that even
the earliest part of the response of ITC neurons could be highly
selective (9).
But even stronger evidence for a feedforward mechanism comes from
another recent study that examined the response of ITC neurons to
strings of images presented in rapid succession (a technique
borrowed from experimental psychology known as RSVP, rapid serial
visual presentation) (10).
Even when the images were changing at 72 Hz (a new image every 14
ms), ITC neurons were still able to follow the input through a
statistically significant modulation of their discharge each time
their preferred stimulus was shown. This kind of data has strong
implications for our understanding of visual processing because it
implies that the visual pathway must be acting as a sort of pipeline
processor, with different images being processed simultaneously at
different levels of the system.
On the other hand, other recent findings suggest that not all
visual information can be analyzed on the basis of this first wave
of information processing. Although the initial response of ITC
neurons is capable of signaling whether a face is present, other
types of information, such as facial expression or identity, are
only available later on (11).
However, in some categorization tasks, the behavioral reaction times
can be so short that the decision is presumably taken without
waiting for this later process to conclude. Freedman et al.
report a mean reaction time of 264 ms, which matches the values seen
with a go/no-go animal categorization task using briefly flashed
photographs (12,
13).
But, when the mean reaction time is 250 to 260 ms, some responses
can be reliably produced with reaction times as short as 180 ms.
This is particularly impressive given that it is only 80 ms longer
than the onset latency of typical ITC neurons. The go/no-go
categorization task is essentially the same task that we have used
to determine the speed of visual processing in humans (14).
Interestingly, monkeys appear to be able to produce behavioral
responses that are substantially faster than those of even the
fastest humans. Although recent replications of the basic scene
categorization task have allowed the shortest reaction times of
human volunteers to be reduced even further, to around 230 to 250 ms
(15),
this value is still roughly 50 ms longer than monkey reaction times.
A number of electrophysiological studies have shown
category-specific activity in humans, but activity onset appears to
be somewhat later than in monkey ITC and PFC neurons. Differential
brain activity between target and nontarget trials has been reported
in human volunteers about 150 ms after stimulus presentation in a
variety of categorization tasks (16).
These include animal versus nonanimal (14,
17,
18)
face versus nonface (19,
20),
and even means of transport (15).
Here again, there are strong grounds for believing that such
category-related activity results from feedforward processing. One
such argument comes from the fact that neither the onset latency of
animal-specific differential activity nor the latency of the
shortest reaction times are any faster for very familiar images
versus images that have never been seen before (21).
Thus, it appears that even extensive contextual information is
unable to increase processing speed--perhaps because neuronal
processing is already so optimized that there is no room for further
improvement.
Could the category-specific activation reported in monkey ITC and
PFC at around 100 ms correspond to the 150-ms activation seen in
humans? Similarities between the monkey and human brain are
difficult to establish, but those between monkey ITC and the more
ventrally located human fusiform gyrus (where much of the
category-related activation seems to be generated) are striking. Why
is it, then, that the onset latencies differ between the two
species? One possible reason is simply that the monkey brain is
smaller than ours. There is not a great deal of detailed information
available, but the conduction velocity of intracortical axons used
to send information from V1 to V2 to V4 to ITC could be relatively
slow, perhaps only 1 to 2 m/s (22).
This means that quite a lot of time may be taken up by simply
getting information from A to B--a problem that is less serious when
your brain is smaller.
But the question still remains whether the category-specific
activity seen in humans corresponds to categorization of the type
described by Freedman et al. in monkeys, in which the
boundaries between categories are specifically coded by single
cells. The alternative is that the strong responses recorded from
structures such as the fusiform gyrus in humans reflect the activity
of large overlapping populations of neurons tuned to particular sets
of objects, as appears to be the case in monkey ITC. The most direct
test requires single-cell recording from individual neurons.
Although normally this is not possible in humans, intracerebral
recording in patients with severe epilepsy recently allowed progress
to be made. For example, recording of individual neurons in the
human medial temporal lobe revealed neuronal responses that were
selective not only for faces, but also for natural scenes, houses,
famous people, and animals (23).
These new data--regardless of whether they represent the rapid
selective visual responses of ITC and PFC neurons in monkeys, the
rapid category-specific signals seen in humans, or the fast
behavioral reaction times seen in both species--pose a major problem
for current models of visual processing. In particular, they imply
that a great deal of processing can be done on the basis of a
largely automatic feedforward pass through the visual system. In a
sense, the fact that visual categorization is fast and robust is
perhaps not so surprising. We all have the impression that as we zap
from channel to channel, the moment when we categorize what the
image contains is virtually instantaneous. The problem now is to
understand how the brain can perform this task so quickly and
efficiently with neurons that fire electrical impulses 10 million
times less rapidly than the transistors in today's desktop
computers.
References
- D. J. Freedman, M. Riesenhuber, T. Poggio, E. K. Miller,
Science 291, 312
(2001).
- C. Bruce et al., J. Neurophysiol.
46, 369 (1981) [Medline].
- D. I. Perrett et al., Exp. Brain Res.
47, 329 (1982) [Medline].
- M. C. A. Booth, E. T. Rolls, Cereb. Cortex
8, 510 (1998) [Medline].
- R. Vogels, Eur. J. Neurosci. 11,
1239 (1999) [Medline].
- D. L. Sheinberg, N. K. Logothetis, J. Neurosci., in
press.
- E. T. Rolls et al., Brain Res.
130, 229 (1977) [Medline].
- E. T. Rolls et al., Brain Res.
164, 121 (1979) [Medline].
- M. W. Oram, D. I. Perrett, J. Neurophysiol.
68, 70 (1992) [Medline].
- C. Keysers et al., J. Cogn. Neurosci., in
press
- Y. Sugase et al., Nature
400, 869 (1999) [Medline].
- A. G. Delorme et al., Vision Res.
40, 2187 (2000) [Medline].
- M. Fabre-Thorpe et al., Neuroreport
9, 303 (1998) [Medline].
- S. Thorpe et al., Nature
381, 520 (1996) [Medline].
- R. Van Rullen, S. J. Thorpe, J. Cogn. Neurosci., in
press.
- A. M. Treisman, N. G. Kanwisher, Curr. Opin.
Neurobiol. 8, 218 (1998) [Medline].
- A. Antal et al., Brain Res. Cogn. Brain Res.
9, 117 (2000) [Medline].
- J. S. Johnson et al., Soc. Neurosci. Abstr.
26, 952 (2000).
- T. Allison et al., Cereb. Cortex
9, 415 (1999) [Medline].
- D. A. Jeffreys, Vis. Cognit. 3, 1
(1996).
- M. Fabre-Thorpe et al., J. Cogn. Neurosci.,
in press.
- L. G. Nowak, J. Bullier, in Extrastriate Cortex in
Primates, J. Kaas, K. Rockland, A. Peters, Eds. (Plenum, New
York, 1997), pp. 205-241 [publisher's
information].
- G. Kreiman et al., Nature Neurosci.
3, 946 (2000) [Medline].
The authors are at the Centre de Recherche Cerveau et Cognition UMR
5549, Université Paul Sabatier, Toulouse, 31062 France. E-mail: thorpe@cerco.ups-tlse.fr
Related articles in Science:
- Categorical Representation of Visual Stimuli in the
Primate Prefrontal Cortex.
- David J. Freedman, Maximilian Riesenhuber, Tomaso Poggio, and
Earl K. Miller
Science 2001 291: 312-316. (in Reports)
[Abstract]
[Full
Text]
Volume 291,
Number 5502, Issue of 12 Jan 2001, pp. 260-263.
Copyright © 2001 by The American Association for the
Advancement of Science.
|
